InCarda Presents Phase 2 Safety and Efficacy Data for InRhythm (Orally Inhaled Flecainide) at the 2022 Heart Rhythm Society Conference in San Francisco
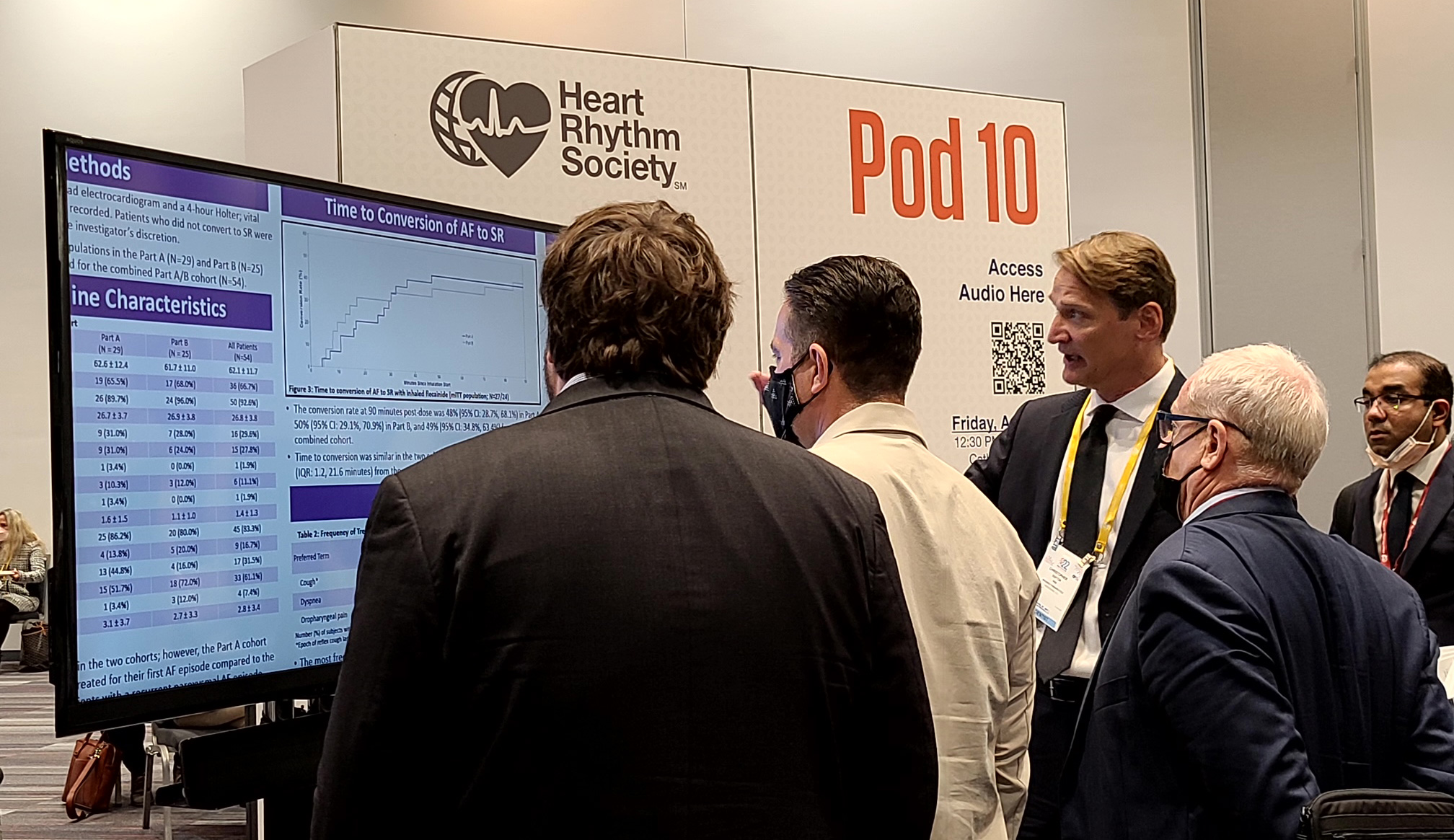
Dr. Chris Dufton, SVP of Clinical Development, answering questions about INSTANT P2 at HRS 2022 in San Francisco.
Phase 2 INSTANT study results demonstrate rapid and reproducible restoration of normal heart rhythm with symptom resolution and a predictable and favorable safety profile for orally inhaled flecainide.
San Francisco, California, May 2, 2022– InCarda Therapeutics, Inc. (InCarda) announced that a poster entitled “Orally Inhaled Flecainide for the Conversion of Recent-Onset, Symptomatic Atrial Fibrillation to Sinus Rhythm – Results from the INSTANT Phase 2 Study” was presented at Heart Rhythm 2022, the annual conference of the Heart Rhythm Society (HRS), an international specialty organization on cardiac pacing and electrophysiology. The analysis presented in this poster includes data from 54 subjects who received the 120mg dose as part of an initial dose-ranging study (Part A; N=29) or as part of a second study (Part B; N=25) that further evaluated this dose level. The combined cohort from the INSTANT Study demonstrated:
- Reproducible results for conversion rate of atrial fibrillation (AF) to sinus rhythm (SR) in the Part A cohort (48%) and Part B cohort (50%)
- A conversion rate of AF to SR of 49% in the combined cohort, with a median time to conversion of 7 minutes from the end of inhalation
- Nearly all patients (96%) who converted to SR reported complete resolution of or improvement in AF symptoms
- Majority (>80%) of treatment emergent adverse events were occurred within 40 minutes of start of dosing and none led to discontinuation of treatment
- Pharmacokinetic and pharmacodynamic data support safety and efficacy profile
Orally inhaled flecainide may provide a safe, effective, and more convenient acute treatment option for episodes of AF compared to either electrical cardioversion or pharmacological cardioversion with IV antiarrhythmic drugs.
Please see here for full poster.
For more information, please visit: https://eventpilotadmin.com/web/page.php?page=Session&project=HRS22&id=P8351