InCarda Presents Additional Phase 2 Data for InRhythm (Orally Inhaled Flecainide) at the 2022 European Society of Cardiology Congress in Barcelona
Phase 2 INSTANT study results demonstrate conversion with inhaled flecainide leads to rapid reduction in ventricular rate and alleviation of atrial fibrillation-related symptoms; the study also demonstrates the potential to reduce healthcare costs related to acute episodes of atrial fibrillation
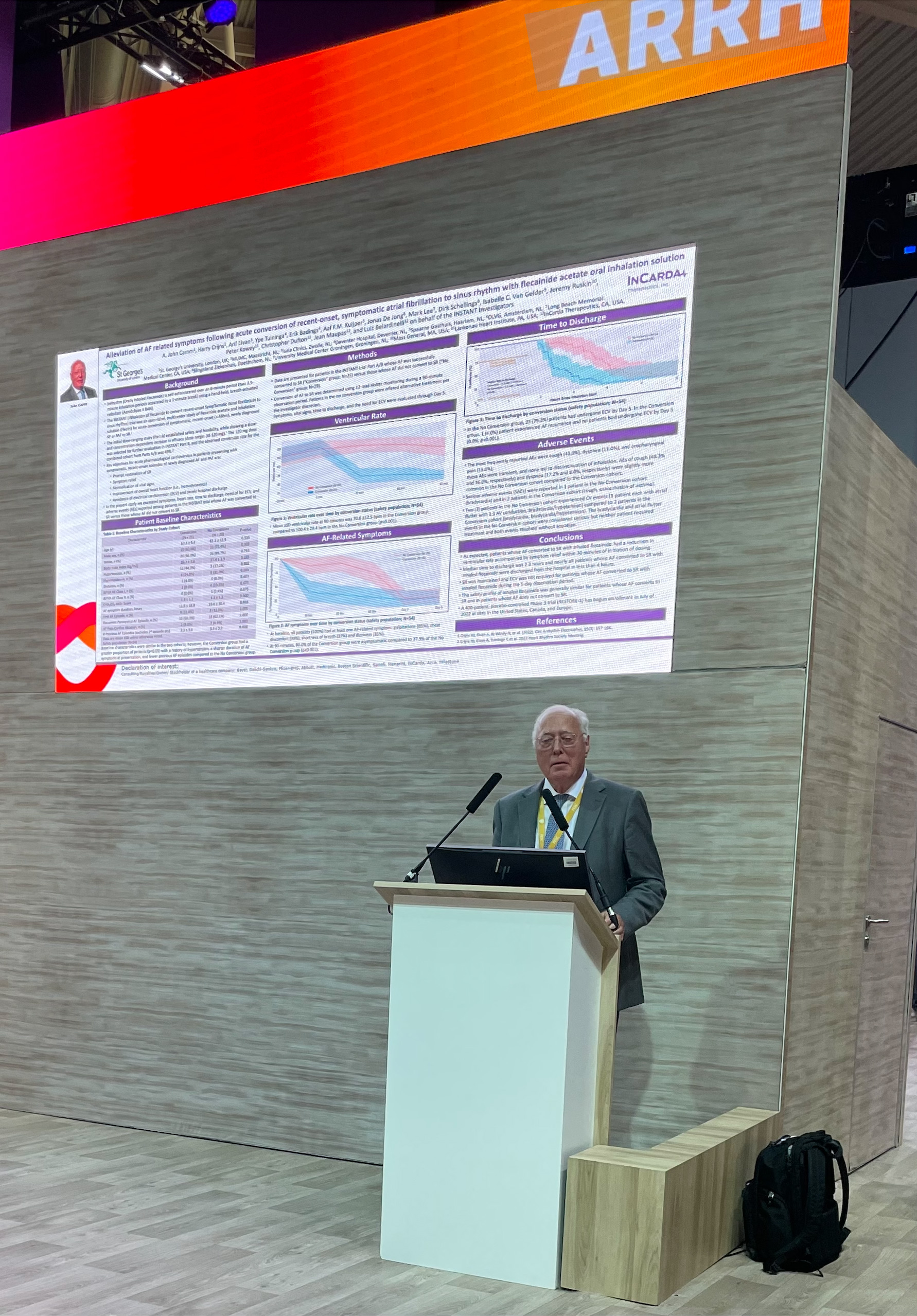
Dr. John Camm presents data from INSTANT Phase 2 at ESC 2022 in Barcelona
San Francisco, California, September 7, 2022 – InCarda Therapeutics, Inc. (InCarda) announced that two posters were presented at the European Society of Cardiology (ESC) Congress, the annual conference of the ESC for clinicians and scientists working in all fields of cardiology. The poster entitled “Alleviation of AF related symptoms following acute conversion of recent-onset, symptomatic atrial fibrillation to sinus rhythm with flecainide acetate oral inhalation solution” was presented by Dr. A. John Camm, MD Professor of Clinical Cardiology at St. Georges University of London, United Kingdom. This study examined the effects of rapid restoration of sinus rhythm with 120 mg dose of orally inhaled flecainide on symptoms, heart rate, time to discharge, need for electrical cardioversion (ECV) and adverse events (AEs) among patients in the Phase 2 INSTANT study. The analysis demonstrated reduction in ventricular rate and accompanying symptoms within 30 minutes in patients whose atrial fibrillation (AF) converted to sinus (SR). Additionally, in these patients, the median time to discharge was less than 2.5 hours and, during the 5-day follow-up, SR was maintained and ECV was not required. These findings suggest the potential of inhaled flecainide to reduce healthcare utilization, including hospitalization, given that more than 50% of Emergency Room visits for AF in the United States require hospitalization1. “Oral inhaled flecainide shows great promise in addressing both clinical and economic unmet needs for the treatment of acute episodes of AF, according to the Phase 2 data presented in Barcelona at the European Society of Cardiology annual congress. There is a need for an effective treatment option to restore sinus rhythm that is faster, safer, and less resource intensive than what is currently available, particularly in the United States” said Dr. Camm who was the first author of the abstract and was a member of the Advisory Committee for the INSTANT trial.
The poster entitled “Predictors of successful cardioversion of recent-onset atrial fibrillation to sinus rhythm with orally inhaled flecainide” was presented by Dr. Harry J.G.M. Crijns, MD, PhD, Professor and Chair of Cardiology at the Cardiovascular Research Institute Maastricht (CARIM) at Maastricht UMC+. The analysis identified body mass index (BMI) to be the strongest predictor of successful cardioversion, which is consistent with results from previous studies2,3. Clinically significant conversion rates were observed across all BMI categories (range: 35% to 55%) except for BMI ≥ 35 kg/m2 (severe obese), though the sample size for this group was small (n=3).
InCarda believes that orally inhaled flecainide may provide a safe, effective, and more convenient acute treatment option for episodes of AF compared to either electrical cardioversion or pharmacological cardioversion with IV antiarrhythmic drugs.
InRhythm (Orally Inhaled Flecainide) is currently being evaluated in a Phase 3 trial (RESTORE-1) to confirm these findings from Phase 2. Study enrollment initiated in July 2022, and more information can be found on ClinicalTrials.gov
Please see here for the full posters.
Reference:
1. Rozen, Ruskin, et al., J Am Heart Assoc. 2018
2. Zeemering S, et al. (2018). Europace. 20(7):e96-e104
3. Ornelas-Loredo et al. (2020). JAMA Cardiol. 5(1):57-64